• Research Articles • Patents • Books • Book Chapters •
Independent Career.
37. Che, W; Lopchuk, J. M. in preparation.
36. Baptiste, C; Shultz, Z. P.; Lopchuk, J. M. in preparation.
35. Shultz, Z. P.; Hall, Y.; Warghude, P. K.; Chang, Y.-P.; Lopchuk, J. M. submitted.
34. Shultz, Z. P.; Lee-Sam, A.; Chang, Y.-P.; Sun, L.; Grassie, D.; Gabellini, A.; Pedretty, K.; Scattolin, T.; Izumi, V.; Fang, B.; Sanil, S.; Kakumanu, R.; Wojtas, L.; Koomen, J.; Schönbrunn, E.; Monastyrskyi, A.; Duckett, D.; Lopchuk, J. M. submitted.
33. Moffitt Team Science collaboration, submitted.
32. Chen, L.; Shultz, Z. P.; Sansone, M.; Fang, B.; Liu, X.; Teng, M.; Schönbrunn, E.*; Lopchuk, J. M.*; Chen, J.* PROTAC-mediated degradation of TAF1 induces apoptosis in AML cells and inhibits tumor growth in vivo. Mol. Cancer Ther. 2025, OF1-OF10.
31. Xing, Q.; Chandrachud, P. P.; Tillett, K.; Lopchuk, J. M. Cobalt-catalyzed radical hydroamination of olefins. Trends Chem. 2025, 7, 202–203.
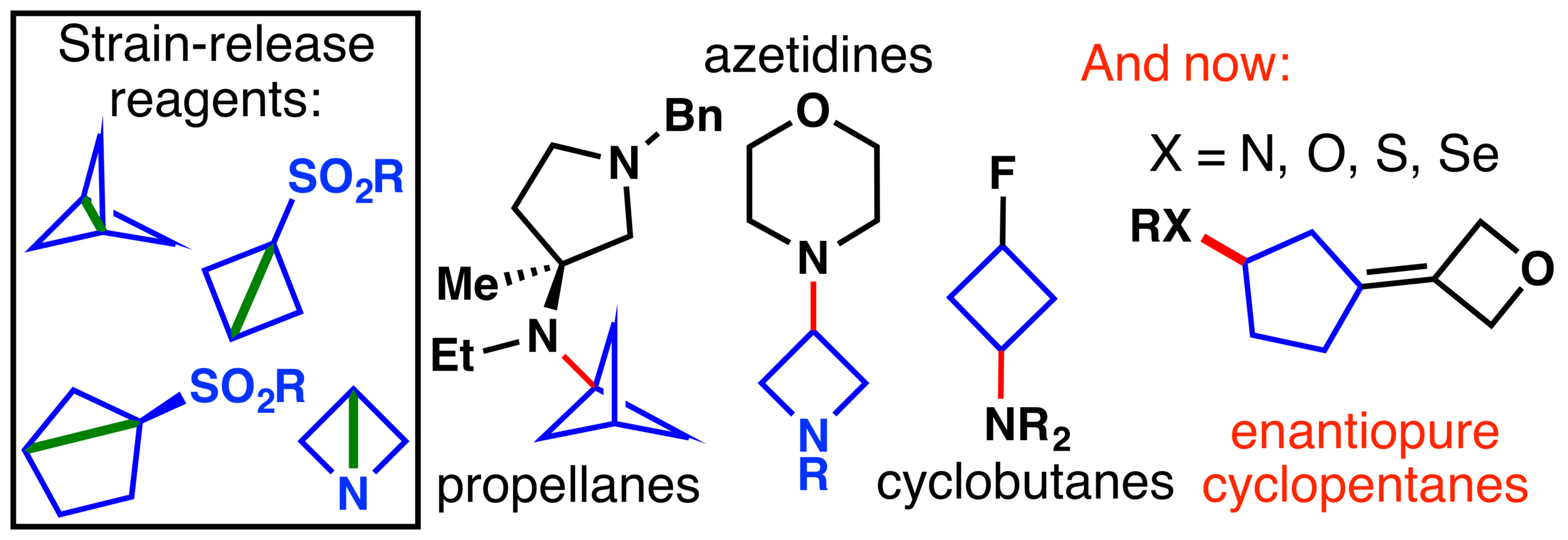
30. Athawale, P.; Shultz, Z. P.; Saputo, A.; Hall, Y.; Lopchuk, J. M. Strain-release driven reactivity of a chiral SuFEx reagent provides stereocontrolled access to sulfinamides, sulfonimidamides, and sulfoximines. Nat. Commun. 2024, 15, 7001. (ChemRxiv version here).
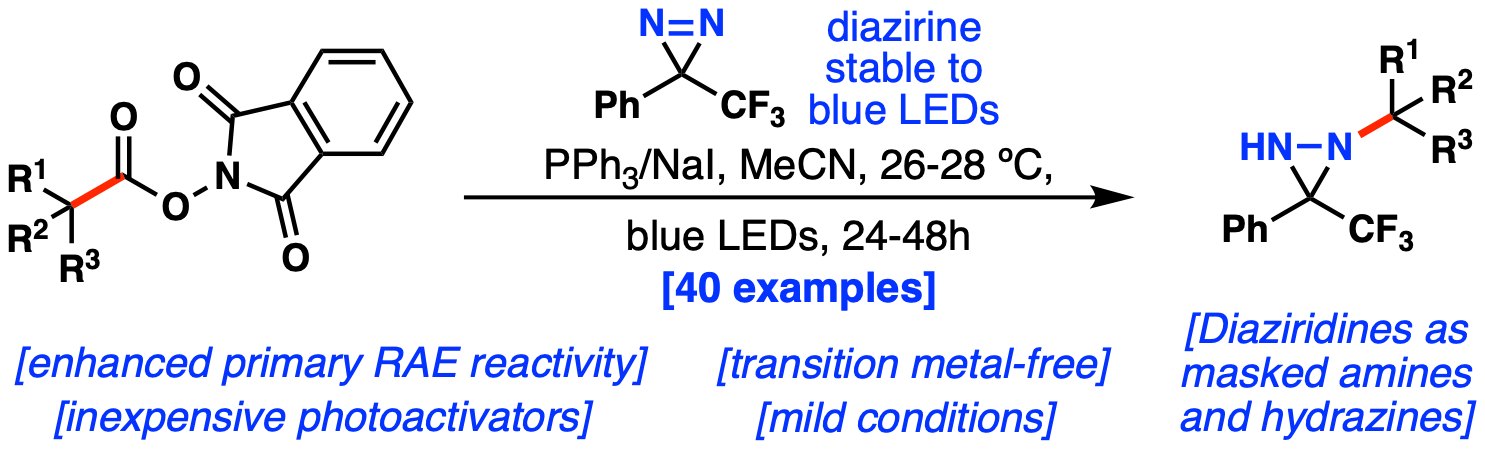
29. Xing, Q.; Chandrachud, P. P.; Tillett, K.; Lopchuk, J. M. Regioselective hydroamination of unactivated olefins with diazirines as a diversifiable nitrogen source. Nat. Commun., 2024, 15, 6049. (ChemRxiv version here).
28. Maharaj, V.; Athawale, P. R.; Chandrachud, P. P.; Lopchuk, J. M. Direct catalytic photodecarboxylative amination of carboxylic acids with diazirines for divergent access to nitrogen-containing compounds. Cell Rep. Phys. Sci. 2024, 5, 102103. (ChemRxiv version here).
27. Che, W; Wojtas, L.; Shan, C.; Lopchuk, J. M. Divergent synthesis of complex withanolides enabled by a scalable route and late-stage functionalization. Sci. Adv. 2024, 10, eadp9375 (ChemRxiv version here).
26. Teng, S.; Shultz, Z. P.; Shan, C.; Wojtas, L.; Lopchuk, J. M. Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides, and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent. Nat. Chem. 2024, 16, 183–192 (ChemRxiv version here)
– Highlighted in Nat. Chem. 2024, 16, 152, by J. A. Bull.
– Highlighted in Synfacts 2024, 20(05), 542, by A. F. Stepan and N. A. Meanwell.
– Highlighted in SYNFORM 2024/06, A99-A101, M. Zanda.
25. Karim, R. M.; Yang, L.; Chen, L.; Bikowitz, M.; Shultz, Z. P.; Lopchuk, J. M.; Chen, J.; Schönbrunn, E. Discovery of dual TAF1-ATR inhibitors and ligand-induced structural changes of the TAF1 tandem bromodomain. J. Med. Chem. 2022, 65, 4182–4200.
24. Shultz, Z. P.; Scattolin, T.; Wojtas, L.; Lopchuk, J. M. Stereospecific α-(hetero)arylation of sulfoximines and sulfonimidamides. Nat. Synth. 2022, 1, 170–179. (ChemRxiv version here).
23. Maharaj, V.; Chandrachud, P. P.; Che, W.; Wojtas, L.; Lopchuk, J. M. Photodecarboxylative amination of redox-active esters with diazirines. Org. Lett. 2021, 23, 8838–8842. (ChemRxiv version here).
22. Shultz, Z. P.; Shan, C.; Wojtas, L.; Lopchuk, J. M. A modular approach for the installation of functionalized phosphonates to heterocycles. Arkivoc 2021, v, 73–96.
– Commemorative issue dedicated to Prof. Peter A. Jacobi on the occasion of his retirement from Dartmouth College. Read the tribute here.
21. Chandrachud, P. P.; Wojtas, L.; Lopchuk, J. M. Decarboxylative amination: Diazirines as single and double electrophilic nitrogen transfer reagents. J. Am. Chem. Soc. 2020, 142, 21743–21750. (ChemRxiv version here).
20. Ji, Y.; Wojtas, L.; Lopchuk, J. M. An improved, gram-scale synthesis of protected 3-haloazetidines: Rapid diversified synthesis of azetidine-3-carboxylic acids. Arkivoc 2018, iv, 195-214.
– Commemorative issue dedicated to Prof. Gordon W. Gribble on the occasion of his retirement from Dartmouth College. Read the tribute here.
Before Moffitt.
19. Lopchuk, J. M.; Baran, P. S. 1-((3,5-Difluorophenyl)sulfonyl)bicyclo[1.1.0]butane. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2017, doi: 10.1002/047084289X.rn02057.
18. Lopchuk, J. M.; Baran, P. S. 1-Azabicyclo[1.1.0]butane. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2017, doi: 10.1002/047084289X.rn02056.
17. Lopchuk, J. M.; Baran, P. S. [1.1.1]Propellane. e-EROS Encyclopedia of Reagents for Organic Synthesis. 2017, doi: 10.1002/047084289X.rn02055.
16. Lopchuk, J. M.; Fjelbye, K.; Kawamata, Y.; Malins, L. R.; Pan, C.-M.; Gianatassio, R.; Wang, J.; Prieto, L.; Bradow, J.; Brandt, T. A.; Collins, M. R.; Elleraas, J.; Ewanicki, J.; Farrell, W.; Fadeyi, O. O.; Gallego, G. M.; Mousseau, J. J.; Oliver, R.; Sach, N. W.; Smith, J. K.; Spangler, J. E.; Zhu, H.; Zhu, J.; Baran, P. S. Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc. 2017, 139, 3209-3226.
– Highlighted in Synfacts 2017, 13, 463, by V. Snieckus and P. Mukherjee.
15. Gianatassio, R.*; Lopchuk, J. M.*; Wang, J.; Pan, C.-M.; Malins, L. R.; Prieto, L.; Brandt, T. A.; Collins, M. R.; Gallego, G. M.; Sach, N. W.; Spangler, J. E.; Zhu, H.; Zhu, J.; Baran, P. S. Strain-Release Amination. Science 2016, 351, 241–246.
– Covered as “News of the Week” in C&E News 2016, 94, 5–6 & Highlighted in RSC’s Chemistry World.
– Highlighted in Nature Chemistry 2016, 8, 296–297, by J. A. Milligan and P. Wipf.
– Highlighted in Synfacts 2016, 12, 352, by V. Snieckus and J.I. Trujillo.
– Highlighted in The Medicine Maker 2016, 0216-202, under the title "Suspended Amination".
14. Cherney, E. C.; Lopchuk, J. M.; Green, J. C.; Baran, P. S. A Unified Approach to ent-Atisane Diterpenes and Related Alkaloids: Synthesis of (–)-Methyl Atisenoate, (–)-Isoatisine, and the Hetidine Skeleton. J. Am. Chem. Soc. 2014, 136, 12592–12595.
– Highlighted in Synfacts 2014, 10, 1120, by E.M. Carreira and H.F. Zipfel.
13. Montgomery, W. L.; Lopchuk, J. M.; Gribble, G. W.; Jasinski, J. P. Synthesis, crystal structures and DFT calculations of three new cyano(phenylsulfonyl)indoles and a key synthetic precursor compound. Crystals 2015, 5, 376–393.
12. Lopchuk, J. M.; Song, M.; Butler, B.; Gribble, G. W. Synthesis of heteroaryl-substituted pyrroles via the 1,3-dipolar cycloaddition of unsymmetrical münchnones and nitrovinyl heterocycles, Synthesis 2015, 47, 2776–2780.
– Invited contribution for thematic issue: Cycloaddition Reactions and Methods.
– Selected by the editorial board for the cover of issue 18/2015.
11. Lopchuk, J. M.; Gribble, G. W. Total synthesis of atorvastatin via a late-state, regioselective 1,3-dipolar münchnone cycloaddition. Tetrahedron Lett. 2015, 56, 3208–3211.
– Invited contribution for Symposium in print: In Memory of Harry Wasserman.
10. Lopchuk, J. M.; Gribble, G. W. The reaction of arynes with münchnones: synthesis of isoindoles and azaisoindoles. Tetrahedron Lett. 2014, 55, 2809–2812.
9. Lopchuk, J. M.; Gribble, G. W.; Jasinski, J. P. Methyl 1-benzyl-5-methyl-2,4-diphenyl-1H-pyrrole-3-carboxylate. Acta Cryst. 2014, E70, o338.
8. Lopchuk, J. M.; Hughes, R. P.; Gribble, G. W. What controls regiochemistry in 1,3-dipolar cycloadditions of münchnones with nitrostyrenes? Org. Lett. 2013, 15, 5218–5221.
7. Lopchuk, J. M.; Montgomery, W.; Jasinski, J. P.; Gorjifard, S.; Gribble, G. W. Manganese(III)-mediated oxidative radical addition of malonates to 2-cyanoindole. Tetrahedron Lett. 2013, 54, 6142–6145.
6. Lopchuk, J. M.; Green, I. L.; Badenock, J. C.; Gribble, G. W. A short, protecting group-free total synthesis of bruceollines D, E, and J. Org. Lett. 2013, 15, 4485–4487.
5. Lopchuk, J. M.; Gribble, G. W.; Jasinski, J. P. Bruceolline D: 3,3-dimethyl-1H,4H-cyclopenta[b]indol-2(3H)-one. Acta Cryst. 2013, E69, o1043.
4. Lopchuk, J. M.; Gribble, G. W.; Millikan, S. P.; Jasinski, J. P. Bruceolline J: 2-Hydroxy-3,3-dimethyl-2,3-dihydrocyclopenta[b]indol-1(4H)-one. Acta Cryst. 2013, E69, o1351–o1352.
3. Lopchuk, J. M.; Gribble, G. W. A convenient 1,3-dipolar cycloaddition approach to pyridylpyrroles. Tetrahedron Lett. 2011, 52, 4106–4108.
– Highlighted in Synfacts 2011, 10, 1054, by V. Snieckus and M. O. Kitching.
2. Lopchuk, J. M.; Gribble, G. W. Synthesis of 2- and 3-indolylpyrroles via 1,3-dipolar cycloadditions of münchnones and nitroalkenes. Heterocycles 2011, 82, 1617–1631.
– Special issue dedicated to Professor Albert Eschenmoser on the occasion of his 85th birthday.
1. Sporn, M. B.; Liby, K. T.; Yore, M. M.; Fu, L.; Lopchuk, J. M.; Gribble, G. W. New synthetic triterpenoids: Potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537–545.
patents.
10. Lopchuk, J. M.; Schönbrunn, E.; Chen, J. Compounds for targeted degradation of TAF1, PCT Int. Appl. WO/2024/057660 filed November 27, 2024.
9. Lopchuk, J. M. Process for synthesis of trifunctionalized sulfur(VI) compounds, PCT Int. Appl. WO/2024/054873 filed November 7, 2024.
8. Lopchuk, J. M. Process for synthesis of withanolides and withaferins and analogs thereof, PCT Int. Appl. WO/2023/014595 filed March 6, 2023, US National 18/844,216 filed September 5, 2024.
7. Lopchuk, J. M.; Shultz, Z. P.; Teng, S. Synthesis of sulfur(VI) compounds and reagents for same, PCT Int. Appl. WO/2023/014592 filed March 6, 2023, US National 18/843,828 filed September 4, 2024.
6. Schönbrunn, E.; Chen, J.; Shultz, Z.P.; Lopchuk, J. M. Compounds for targeted degradation of TAF1, PCT Int. Appl. WO/2022/076277 filed September 12, 2022, US National 18/690,403 filed March 8, 2024.
5. Schönbrunn, E., Chen, J., Karim, M. R., Lopchuk, J. M., Shultz, Z. P. Merged scaffold TAF1 inhibitors, PCT Int. Appl. WO/2021/061121 filed November 30, 2021, US National 18/039,386 filed May 30, 2023.
4. Lopchuk, J. M.; Shultz, Z. P.; Scattolin, T. Process for the preparation of heteroaryl-substituted sulfur(VI) compounds, PCT Int. Appl. WO/2021/057262 filed October 29, 2021, US National 18/034,439 filed April 28, 2023.
3. Schönbrunn, E., Lopchuk, J. M., Karim, M. R., Shultz, Z. TAF1 inhibitors, PCT Int. Appl. WO/2021/189036 filed March 22, 2021, US National 17/913,033 filed September 20, 2022.
2. Lopchuk, J. M.; Chandrachud, P. P. Processes and compounds for the decarboxylative amination of redox-active esters with diazirines, PCT Int. Appl. WO/2020/252457, June 15, 2020, US National 17/617,973 filed December 10, 2021.
1. Gribble, G. W.; Lopchuk, J. M. Compositions and methods for treating cancer, U.S. Patent Application, 20120022097, September 20, 2011; US Patent 8,975,274 issued March 10, 2015.
books.
2. Progress in Heterocyclic Chemistry, Volume 37; Aiken, R. A., Lopchuk, J. M., Eds.; Elsevier: Amsterdam, commissioned, in preparation.
1. Progress in Heterocyclic Chemistry, Volume 36; Aiken, R. A., Lopchuk, J. M., Eds.; Elsevier: Amsterdam, 2024, 517 pp.
book chapters.
19. Bhowmick, A.; Galaktionova, D.; Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Aiken, R. A.; Lopchuk, J. M., Eds.; Elsevier Science: New York, 2025, 37, submitted.
18. Shan, C.; Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Aiken, R. A.; Lopchuk, J. M., Eds.; Elsevier Science: New York, 2024, 36, 123–173.
17. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Aiken, R. A., Eds.; Elsevier Science: New York, 2024, 35, 199–250.
16. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Aiken, R. A., Eds.; Elsevier Science: New York, 2023, 34, 155–207.
15. Shultz, Z. P.; Lopchuk, J. M. Strategies for the synthesis of sulfoximine-containing heterocycles. In Advances in Heterocyclic Chemistry. Scriven, E. F. V.; Ramadan, C. A. 2022, 138, 61–158.
14. Tillett, K.; Pedretty, K.; Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2021, 33, 119–173.
13. Pedretty, K.; Tillett, K.; Tsuei, W.; Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2021, 32, 193–240.
12. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2020, 31, 223–280.
11. Lopchuk, J. M. Imide Natural Products. In Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry (Developments in Organic Chemistry), Luzzio, F. A., Ed.; Elsevier: New York, 2019, 255–334.
10. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2018, 30, 111–168.
9. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2017, 29, 183–238.
8. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2016, 28, 165–218.
7. Gianatassio, R.; Lopchuk, J. M.* Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2015, 27, 159–202.
6. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2014, 26, 151–192.
5. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2013, 25, 137–182.
4. Lopchuk, J. M. Pyrroles and Benzo Derivatives. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2012, 24, 169–204.
3. Lopchuk, J. M. Mesoionics. In Topics in Heterocyclic Chemistry. Gribble G.W., Ed.; Metalation of Azoles and Related Five-Membered Ring Heterocycles; Springer-Verlag: Berlin, 2012, 29, 381–413.
2. Lopchuk, J. M. Azoles with 3-4 Heteroatoms. In Topics in Heterocyclic Chemistry. Gribble G.W., Ed.; Metalation of Azoles and Related Five-Membered Ring Heterocycles; Springer-Verlag: Berlin, 2012, 29, 415–440.
1. Lopchuk, J. M. Recent advances in the synthesis of Aspidosperma-type alkaloids. In Progress in Heterocyclic Chemistry, Gribble, G. W.; Joule, J. A., Eds.; Elsevier Science: New York, 2011, 23, 1–25.